Introduction: Looking Beyond Price in Orthopedic Procurement 🏥
In global orthopedic procurement, purchasing decisions are rarely made on price alone. Increasing regulatory complexity, tighter hospital budgets, and higher expectations for clinical reliability have pushed decision-makers to adopt a more comprehensive evaluation model. Total Cost of Ownership orthopedic devices has therefore become a practical and strategic framework for hospitals, distributors, and healthcare systems worldwide.
At AMIS Orthopedic, procurement discussions often begin with a simple but critical question: How will this system perform financially, operationally, and clinically over its entire lifecycle? Total Cost of Ownership provides the structure to answer that question with clarity and confidence.
Understanding Total Cost of Ownership in Orthopedic Systems 🔍
Total Cost of Ownership orthopedic devices refers to all costs incurred from acquisition through daily use and eventual replacement. This includes not only the initial purchase price, but also maintenance, sterilization, staff training, downtime, and regulatory compliance.
A structured orthopedic device TCO analysis helps procurement teams compare systems on long-term value rather than short-term cost. This approach is particularly relevant for reusable orthopedic systems such as surgical power tools, where operational expenses accumulate steadily over years of use.
Device Longevity as a Foundation of TCO Value 🛠
Durability plays a central role in any realistic TCO assessment. Orthopedic devices are exposed to repeated mechanical stress, vibration, and high-temperature sterilization cycles. Systems engineered for long-term reliability reduce replacement frequency and stabilize budgeting.
AMIS Orthopedic designs surgical power systems to withstand repeated 135°C autoclave sterilization cycles without compromising performance. Over time, this durability directly reduces the surgical power tool lifecycle cost, lowering cost per procedure and minimizing unexpected capital expenditures.
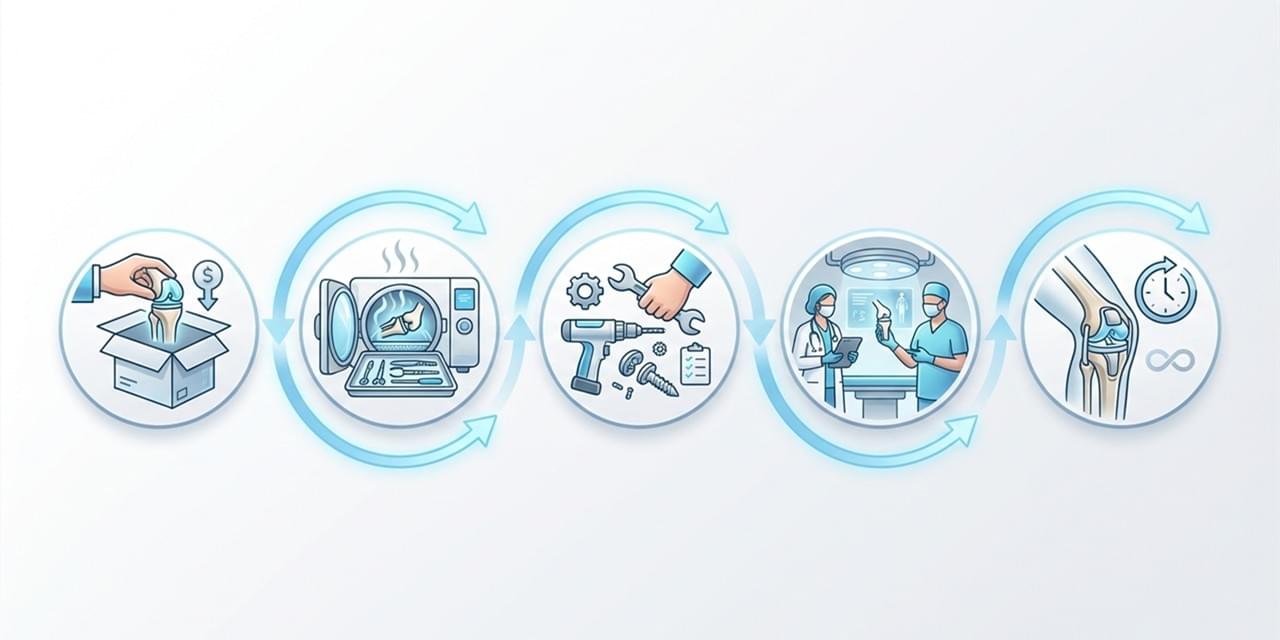
Maintenance Efficiency and Operational Continuity 🔧
Maintenance-related costs often represent the most underestimated component of Total Cost of Ownership. Devices that require frequent servicing or specialized spare parts increase both direct repair costs and indirect downtime risks.
From a Total Cost of Ownership orthopedic devices perspective, modular system architecture, standardized couplings, and predictable service intervals are critical. These features support faster maintenance, easier troubleshooting, and uninterrupted surgical schedules—key priorities for both hospitals and distributors.
Sterilization Workflow and Reprocessing Impact ♻
Central Sterile Services Departments (CSSD) significantly influence long-term device economics. Complex instrument designs that are difficult to clean increase labor time, reprocessing errors, and the likelihood of premature wear.
Orthopedic systems designed for smooth surfaces, easy disassembly, and compatibility with standard hospital detergents reduce cumulative sterilization costs. This operational efficiency is an essential element of effective orthopedic procurement cost management, particularly in high-volume surgical environments.
Training, Standardization, and System Compatibility 🎓
Training requirements directly affect adoption speed and operational efficiency. Systems that align with established surgical workflows shorten learning curves and reduce procedural variability.
AMIS Orthopedic emphasizes system compatibility and standardization to support multi-department use. Devices that integrate seamlessly with existing accessories and hospital infrastructure help procurement teams reduce inventory complexity while optimizing Total Cost of Ownership orthopedic devices across departments.
Regulatory Transparency and Risk Control 📋
Regulatory risk is an often overlooked but costly TCO factor. Devices lacking complete technical documentation or quality system alignment can trigger audits, corrective actions, or delayed approvals.
AMIS Orthopedic operates under ISO 13485-certified quality management systems
(https://www.iso.org/standard/59752.html) and aligns product documentation with global regulatory expectations, including FDA guidance
(https://www.fda.gov/medical-devices). This transparency reduces compliance-related uncertainty and protects long-term procurement investments.
Supply Chain Stability and Long-Term Partnership Value 🤝
Supply continuity and contractual clarity strongly influence lifecycle cost. Unstable suppliers may offer lower initial prices, but introduce risks through delayed deliveries, inconsistent service, or unclear warranty terms.
AMIS Orthopedic positions itself as a long-term manufacturing and distribution partner, offering predictable lead times, clear service agreements, and technical continuity. More information on AMIS orthopedic power systems can be found at:
👉https://www.orthopdevices.com/product-category/surgical-power-tools/
Such stability supports strategic sourcing and lowers the total financial exposure over the product lifecycle.
Total Cost of Ownership as a Strategic Lens 📊
Total Cost of Ownership orthopedic devices reframes procurement from a transactional activity into a strategic decision-making process. By evaluating durability, maintenance efficiency, sterilization compatibility, training requirements, regulatory transparency, and supply stability, procurement leaders gain a realistic view of long-term value.
For AMIS Orthopedic, TCO is not a marketing concept—it is a practical tool that aligns engineering discipline, clinical reliability, and procurement accountability. In an increasingly complex healthcare environment, TCO-driven decisions support sustainable orthopedic programs that deliver consistent performance today and long into the future.